Extraforaminal needle tip position reduces risk of intravascular injection in CT-fluoroscopic lumbar transforaminal epidural steroid injections
Introduction
Thoracolumbar transforaminal epidural steroid injections (TFESIs) are an effective short-term nonsurgical treatment option for managing chronic lumbar radicular spinal pain (1). The procedure is common, with over 1,100,000 injections performed on Medicare patients alone in 2011 (2).
Paralysis is a rare but catastrophic complication of thoracolumbar TFESI (3). It is most commonly thought to be caused by accidental needle tip penetration of the artery of Adamkiewicz within the targeted neural foramen and subsequent embolization of particulate steroid into the anterior spinal artery (4-6). There has been great interest in developing procedural techniques to reduce the risk of intra-arterial injection of steroid (7-10).
For cervical CT-fluoroscopic TFESIs, previous literature has shown that the extraforaminal needle tip position correlates to lower incidence of intravascular injection (11). We now investigate whether a similar correlation exists for lumbar TFESIs.
The purpose of this study is to determine the safest needle tip position for CT-guided lumbar TFESIs as determined by incidence of intravascular injection. We also characterize intravascular injections by: vessel type injected, volume of the intravascular injection, and whether intravascular injection occurred with contrast trial injection, steroid/analgesic cocktail injection, or both.
Methods
Patient population
Local institutional review board approval was obtained for this retrospective review of clinical and imaging data. This study was compliant with the Health Insurance Portability and Accountability Act.
We retrospectively searched our radiology information system for all consecutive CT-fluoroscopic guided lumbar TFESIs performed by members of our neuroradiology section at our main academic campus, during a 16-month period (February 10, 2014 to June 30, 2015).
Our goal of characterizing needle tip position relative to neural foraminal landmarks required relatively constant morphology among targeted neural foramina. We therefore chose to exclude TFESIs targeting thoracic or sacral neural foramina. We also excluded L5-S1 TFESIs when there was sacralization of the L5 vertebra (which created a targeted L5-S1 neural foramen resembling a sacral, not lumbar, neural foramen). If a patient underwent both lumbar and non-lumbar TFESIs in the same procedural setting, only the lumbar TFESI were considered for this study. We also excluded lumbar TFESIs not utilizing radiopaque contrast.
Procedural technique
All procedures were performed by 1 of 3 academic neuroradiologists with the Certificate of Added Qualification in Neuroradiology and having 4 (GML), 8 (VA), or >20 years experience performing image-guided spine procedures.
The injections were performed as previously described (12) with the following additional details: all procedures were performed on the same GE LightSpeed Plus 4-detector row CT scanner (GE Healthcare, Milwaukee, Wisconsin). After acquisition of a short-length CT scan for planning purposes, intermittent intraprocedural CT-fluoroscopic imaging was acquired using SmartView (GE Healthcare, Milwaukee, Wisconsin) triggered by a foot pedal. Each acquisition created 3 consecutive axial images with the following parameters: section thickness 2.5 mm, helical rotation time 0.8 seconds, speed 75 mm/rotation, pitch 0.75:1, 120 kV, and variable mA. All planning and CT-fluoroscopic imaging from the procedure was automatically archived to our hospital PACS, so that we were able to evaluate all imaging acquired at the time of the procedure during our retrospective review.
Quincke-tipped spinal needles (BD Medical, Franklin Lakes, New Jersey) of a variety of calibers (25, 23, and 22 gauge) and lengths [3.5 inch (90 mm), 5 inch (130 mm), and 7 inch (180 mm)] were utilized for the procedures. Using intermittent CT-fluoroscopic guidance, needles were positioned at or near the posterior aspect of the neural foramen.
With short-length flexible microbore attached to the needle hub, a trial dose was injected using 0.3 mL of iohexol contrast agent (Omnipaque, 180 mg/mL; GE Healthcare, Piscataway, New Jersey). CT-fluoroscopic imaging was acquired immediately after trial dose injection and scrutinized for intravascular contrast. If intravascular contrast was identified, the needle was then withdrawn a few millimeters, a repeat trial dose injection of 0.3 mL iohexol contrast was performed with repeat CT-fluoroscopic imaging. These steps were repeated until no additional intravascular contrast was identified.
A cocktail of 80 mg methylprednisolone steroid and 2.0 mL of preservative-free 2.5 or 5 mg/mL bupivacaine analgesic was then injected under additional intermittent fluoroscopic imaging into or near the targeted neural foramen. We did not specifically mix contrast with our steroid/analgesic cocktail, although we did inject the steroid/analgesic cocktail through the same needle and microbore tubing used immediately previous for the contrast trial dose injection without intervening tubing flush. “Dead space” residual contrast within the microbore tubing was therefore injected at the beginning of the steroid/analgesic contrast injection. With this technique, we were able to evaluate for intravascular injection with the steroid/analgesic injection.
Following the procedure, the patient was monitored for 15 minutes to evaluate for minor complications (such as vasovagal response or increasing pain) or major complications (such as cardiovascular or neurologic compromise). Complications were appropriately treated and later reported in the formal procedural report by the attending physician.
Imaging evaluation
All imaging was evaluated by two of the proceduralists (GML and VA) and a post-graduate-year 4 radiology resident (RKU), all of whom were blinded to operator and patient identity. The 3 reviewers evaluated and characterized all imaging separately. In the case of disagreement, the relevant imaging was re-evaluated in a group setting and a consensus achieved regarding findings and characterization.
Needle tip position
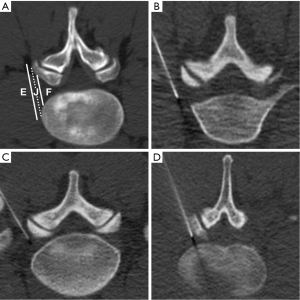
Our classification of needle tip position is shown in Figure 1. All injections were categorized by needle tip position relative to the targeted lumbar neural foramen at the time of injection using a three-part categorization similar to a scheme previously described for cervical TFESIs (11,13,14).
Intravascular injection
An intravascular injection were considered to be present if 1 of 2 contrast appearances was identified on CT-fluoroscopy, similar to previously-described criteria (11,15):
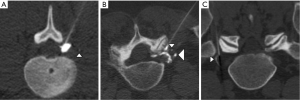
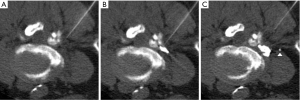
- Contrast appearing as ≥1 discrete round or curvilinear foci, in a morphology consistent with a vessel, and separate from the dominant accumulated epidural contrast collection and needle tip (Figure 2A,B). Washout of contrast on subsequent CT-fluoroscopic imaging helped to confirm, but was not required to establish, intravascular injection. In this situation, contrast was assumed to be opacifying small vessels within the CT-fluoroscopy’s field of view;
- CT-fluoroscopic imaging acquired immediately after injection clearly showed less-than-expected volume of accumulated epidural contrast, or no epidural contrast accumulation at all (Figure 2C). In this situation, the missing contrast was assumed to have been injected into a vessel and already rapidly circulated out of the field of view at the time of CT-fluoroscopic imaging. Subsequent appropriate epidural accumulation of contrast after needle adjustment helped to confirm, but was not required to prove, the intravascular injection.
Intravascular injection and vessel type
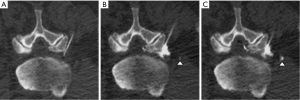
Intravascular injections were classified by likely vessel type into one of three categories (Figure 3):
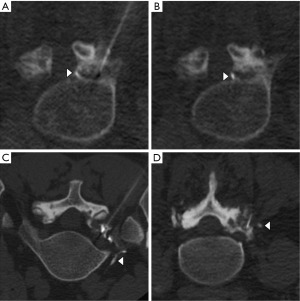
- Likely arterial (Figure 3A,B): punctate or curvilinear opacification of a vessel in the anterior superior neural foramen along the superior margin of the foraminal nerve root, which represents the most common location of a radiculomedullary artery (including the artery of Adamkiewicz);
- Likely venous (Figure 3C): curvilinear vessel opacification extending anteriorly from the paraspinal region toward an iliac vein or the inferior vena cava;
- Indeterminate (Figure 3D): all intravascular injections that were not clearly arterial or venous. These included most trace-volume paraspinal intravascular injections and most large-volume intravascular injections when neither enhancing radiculomedullary arteries nor draining veins were clearly identified.
Intravascular injection volume
Intravascular injections were classified by volume into one of three categories (Figure 2):
- Trace (Figure 2A): 1–2 discrete tiny foci of contrast, each measuring ≤2 mm in transaxial dimension, and clearly separate from the needle tip and its associated dominant epidural contrast collection;
- Small (Figure 2B): either ≥3 discrete foci of contrast, or ≥1 foci of contrast measuring ≥3 mm in transaxial dimension, all separate from the needle tip and its dominant associated dominant epidural contrast collection. However, the volume of the dominant epidural contrast collection was not clearly smaller than expected;
- Large (Figure 2C): a clearly smaller-than-expected volume of accumulated epidural contrast adjacent to the needle tip, or no identified contrast at all. In this situation, the injection was interpreted to be mostly or completely intravascular, with most or all of the intravascular contrast already circulated out of the CT-fluoroscopic field of view.
Intravascular injection and procedural phase
Intravascular injections were classified by procedural phase into one of three categories (Figures 4-6):
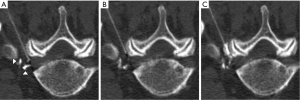
- Contrast trial dose (Figure 4): intravascular contrast was identified on the initial CT-fluoroscopic imaging acquired, immediately after contrast trial dose injection. Evidence of subsequent needle tip repositioning, either described by the operator in the procedural report or directly identified on careful retrospective evaluation of procedural imaging, combined with no evidence of further intravascular injection, together confirmed that there was no steroid/analgesic intravascular injection;
- Intravascular injection of steroid/analgesic cocktail (Figure 5): intravascular contrast was not present with the initial contrast trial dose injection, even on careful retrospective evaluation of imaging. However, intravascular injection was present on later imaging corresponding to the steroid/analgesic cocktail injection;
- Both contrast trial injection and steroid/analgesic cocktail injection (Figure 6): intravascular injection was present with the initial contrast trial injection. Also, either additional intravascular contrast was clearly identified following the steroid/analgesic cocktail injection, or there was no evidence of subsequent needle repositioning either in the procedural report or on careful retrospective analysis of procedural imaging. Because the 2 components of intravascular injection were closely linked and intimately related, we considered this situation to represent a single intravascular injection with components in two phases.
Statistical testing
Pearson chi-square testing was used to assess differences in vascular injections based on needle position. Differences in vascular injections were assessed on the basis of age, sex, and prior surgical history by using logistic regression and Pearson chi-square testing as appropriate. If appropriate, post hoc multiple comparison testing was performed. Statistical testing was performed using JMP 11 (SAS Institute, Cary, North Carolina, USA).
Results
Patient population
A total of 431 patients underwent 606 lumbar TFESIs in the setting of 538 procedural encounters, most of which were single-level injections. Based on procedural level, 32 thoracic TFESIs, 143 sacral TFESIs, and 2 L5–S1 TFESIs involving sacralized L5 vertebrae were excluded from the study.
The mean patient average age was 57 years (range, 14–88 years). 49% (210/431) of patients were male; 51% (221/431) were female. The most frequently targeted neural foraminal levels were L5–S1 (52%, 314/606) and L4–L5 (28%, 170/606). There were no significant differences in age, sex, and level of injection among groups based on intravascular injection (P>0.05).
Procedural needle type
In regards to procedural needle caliber, 67% (405/606) of TFESIs were performed with a 25-gauge needle, 27% (164/606) with a 23-gauge needle, and 6% (37/606) with a 22-gauge needle. For procedural needle length, 62% (375/606) of TFESIs used a 3.5-inch needle, 35% (215/606) a 5-inch needle, and 3% (16/606) a 7-inch needle. There were no significant differences in needle caliber and length when comparing presence or absence of intravascular injection (P>0.05).
Needle tip position and intravascular injection
For all included lumbar TFESIs, needle tip position was extraforaminal in 18% (109/606), junctional in 53% (319/606), and foraminal in 29% (178/606) of injections.
Intravascular injection was identified in 9% (52/606) of lumbar TFESIs. Intravascular injection rate was significantly lower for extraforaminal needle tip position (0%, 0/109) compared to junctional (8%, 27/319) and foraminal (14%, 25/178) needle tip positions (pair-wise comparisons: extraforaminal versus junctional, P<0.001; extraforaminal versus foraminal, P<0.001; junctional versus foraminal, P=0.07).
Intravascular injection characterization
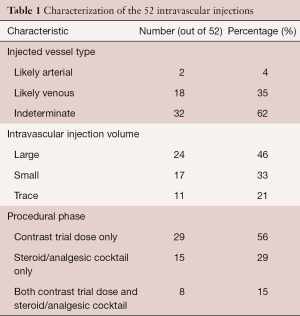
Characterization of the 52 intravascular injections is given in Table 1.
Full table
In regards to vessel type injected, likely arterial injections were least common (4%), likely venous more common (35%), and injections indeterminate as to vessel type injected were most common (62%). The 2 likely arterial injections occurred with a foraminal needle tip position.
Almost half (46%) of intravascular injections were large volume, 33% were small volume, and 21% were trace volume. Most large-volume injections occurred with the contrast trial dose only. All trace-volume injections were indeterminate in regards to vessel type injected.
Of the intravascular injections, the majority (56%) were present on the contrast trial dose injection only, 29% with the steroid/analgesic cocktail injection only, and 15% with both the contrast trial dose injection and the steroid/analgesic cocktail injection. All intravascular injections with both contrast trial dose and steroid/analgesic components had the same volume of intravascular injection in the two components.
Complications
For the 606 included lumbar TFESIs, 1 minor complication was reported: accidental advancement of the needle tip into the thecal sac and subsequent intrathecal injection of steroid/analgesic cocktail resulting in 2 hours of bilateral lower extremity weakness and paresthesias. The patient was monitored in a hospital outpatient recovery unit and was sent home after the symptoms had resolved. There were no complications attributable to accidental intravascular injection.
Discussion
Intravascular injections were identified in 9% of our lumbar TFESIs. Intravascular injections were less likely for the extraforaminal needle tip position compared to junctional or foraminal needle tip position (P<0.001 for both pairwise comparisons). Likely arterial injections were least common (4%) compared to likely venous (35%) and indeterminate vessel injections (62%).
We have shown that extraforaminal needle tip position correlates to a lower incidence of intravascular injection. An extraforaminal needle tip may therefore reduce risk for spinal cord infarction and represent a relatively safe needle tip position for lumbar TFESIs.
Our overall CT-fluoroscopic lumbar TFESI intravascular injection incidence of 9% is very close to the 8% incidence reported by Kranz et al. (15), although that group identified all intravascular injections with the contrast trial injection. To our knowledge, we are the first to observe, in the lumbar spine, the direct CT-fluoroscopic imaging evidence of intravascular injection of steroid.
Accidental injection of particulate steroid into the artery of Adamkiewicz, with embolization to the anterior spinal artery, is the most commonly-cited cause of spinal cord injury following lumbar TFESI (4-6,16), although direct needle injury of the artery of Adamkiewicz has also been suggested as a cause (4,16). The specific neural foraminal level and laterality of the artery of Adamkiewicz is highly variable: it most often originates between the T9 and L3 levels and usually on the left, but origins from as high as the T2 level and as low as the S2 level have been described (17-22). In practice, the proceduralist must assume that the artery of Adamkiewicz could be present in any thoracic, lumbar, or upper sacral neural foramen.
There are 19 case reports of spinal cord injury following conventional fluoroscopic- or CT-guided thoracolumbar TFESI (3,4,6,8,16,23-29) [(although the complication is known to be underreported due to its medicolegal implications (8)]. Review of the imaging and procedural descriptions for these 19 case reports shows a foraminal needle location, sometimes deep within the neural foramen, in 7 of these cases; in the remaining 12, insufficient information is provided to determine needle depth relative to the targeted neural foramen (3,29). The preponderance of foraminal needle tip positions in these cases suggests a correlation between foraminal needle tip position and spinal cord injury, although alternatively this could represent the fact that foraminal needle tip position may be the most frequently-used technique for lumbar TFESI.
Great concern has been expressed in the literature regarding spinal cord injury as a devastating and unacceptable complication from lumbar TFESI (30), with the proposal of many procedural techniques that might protect from spinal cord injury. The fluoroscopic literature commonly describes the “safe” triangle, a target in the anterior superior neural foramen intended to avoid injuring the nerve root (31). However, this targeted region is by far the most likely location of the radicular artery within the neural foramen, and the “safe” triangle appears to be actually the least safe target in the neural foramen (8,21,24,32). An alternative, putatively safer target called “Kambin’s triangle,” (8,33,34) in the inferior posterior neural foramen has been described, although this region also often contains arterial vessels (32). Use of some adjunct techniques, such as digital subtraction angiography with fluoroscopic-guided lumbar TFESI (7) and evaluation for bloody “flash” in the needle hub may be useful, although accidental intravascular injections have nevertheless been described despite the use of these techniques (28,35,36). The use of Whitacre needles (10) and non-particulate steroids (37) has also been promoted.
Over one-third of our intravascular injections were likely venous. The clinical relevance of accidental intravenous injection of steroid/analgesic cocktail in this situation is poorly understood. At a minimum, a large-volume intravenous injection of steroid would be expected to diminish or completely remove the intended concentrated local anti-inflammatory effect of the steroid, and instead convert the injection into an unintended low-dose intravenous steroid injection. In addition, some authors have speculated that accidental intravenous injection of corticosteroid is the cause of Tachon syndrome: excruciating thoracic or low back pain with dramatic, abrupt onset within a few minutes of a local steroid injection and rapid resolution, with a mean duration of 25 minutes. Incidence is estimated at 1 in 8,000 local steroid injections (38-40). Given the possible risks of intravenous injection, we suggest the proceduralist make efforts to avoid venous as well as arterial steroid injections.
The limits of this study include those inherent in a retrospective, single-institution review. In addition, we do not have follow-up pain relief data for these injections, so the possibility that extraforaminal needle tip position decreases diagnostic and therapeutic efficacy of the procedure keeps us from suggesting that the extraforaminal zone is the preferred needle tip position for lumbar TFESIs. A follow-up study correlating needle tip position and pain relief would be useful to address these questions.
In conclusion, extraforaminal needle tip position correlates to lower incidence of intravascular injection and may represent a safer needle tip position for lumbar TFESIs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Local institutional review board approval was obtained for this retrospective review of clinical and imaging data. This study was compliant with the Health Insurance Portability and Accountability Act.
References
- Manchikanti L, Buenaventura RM, Manchikanti KN, et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician 2012;15:E199-245. [PubMed]
- Manchikanti L, Pampati V, Falco FJ, et al. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 2013;16:E349-64. [PubMed]
- Atluri S, Glaser SE, Shah RV, et al. Needle position analysis in cases of paralysis from transforaminal epidurals: consider alternative approaches to traditional technique. Pain Physician 2013;16:321-34. [PubMed]
- Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J 2002;2:70-5. [Crossref] [PubMed]
- Tiso RL, Cutler T, Catania JA, et al. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J 2004;4:468-74. [Crossref] [PubMed]
- Lyders EM, Morris PP. A case of spinal cord infarction following lumbar transforaminal epidural steroid injection: MR imaging and angiographic findings. AJNR Am J Neuroradiol 2009;30:1691-3. [Crossref] [PubMed]
- Jasper JF. Role of digital subtraction fluoroscopic imaging in detecting intravascular injections. Pain Physician 2003;6:369-72. [PubMed]
- Glaser SE, Shah RV. Root cause analysis of paraplegia following transforaminal epidural steroid injections: the 'unsafe' triangle. Pain Physician 2010;13:237-44. [PubMed]
- MacMahon PJ, Shelly MJ, Scholz D, et al. Injectable corticosteroid preparations: an embolic risk assessment by static and dynamic microscopic analysis. AJNR Am J Neuroradiol 2011;32:1830-5. [Crossref] [PubMed]
- Hong J, Jung S, Chang H. Whitacre Needle Reduces the Incidence of Intravascular Uptake in Lumbar Transforaminal Epidural Steroid Injections. Pain Physician 2015;18:325-31. [PubMed]
- Lagemann GM, Yannes MP, Ghodadra A, et al. CT-Fluoroscopic Cervical Transforaminal Epidural Steroid Injections: Extraforaminal Needle Tip Position Decreases Risk of Intravascular Injection. AJNR Am J Neuroradiol 2016;37:766-72. [Crossref] [PubMed]
- Wagner AL. Selective lumbar nerve root blocks with CT fluoroscopic guidance: technique, results, procedure time, and radiation dose. AJNR Am J Neuroradiol 2004;25:1592-4. [PubMed]
- Wolter T, Knoeller S, Berlis A, et al. CT-guided cervical selective nerve root block with a dorsal approach. AJNR Am J Neuroradiol 2010;31:1831-6. [Crossref] [PubMed]
- Hoang JK, Massoglia DP, Apostol MA, et al. CT-guided cervical transforaminal steroid injections: where should the needle tip be located? AJNR Am J Neuroradiol 2013;34:688-92. [Crossref] [PubMed]
- Kranz PG, Amrhein TJ, Gray L, et al. Incidence of inadvertent intravascular injection during CT fluoroscopy-guided epidural steroid injections. AJNR Am J Neuroradiol 2015;36:1000-7. [Crossref] [PubMed]
- Huntoon MA, Martin DP. Paralysis after transforaminal epidural injection and previous spinal surgery. Reg Anesth Pain Med 2004;29:494-5. [Crossref] [PubMed]
- Lazorthes G, Gouaze A, Zadeh JO, et al. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. J Neurosurg 1971;35:253-62. [Crossref] [PubMed]
- Alleyne CH Jr, Cawley CM, Shengelaia GG, et al. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg 1998;89:791-5. [Crossref] [PubMed]
- Biglioli P, Spirito R, Roberto M, et al. The anterior spinal artery: the main arterial supply of the human spinal cord--a preliminary anatomic study. J Thorac Cardiovasc Surg 2000;119:376-9. [Crossref] [PubMed]
- Biglioli P, Roberto M, Cannata A, et al. Upper and lower spinal cord blood supply: the continuity of the anterior spinal artery and the relevance of the lumbar arteries. J Thorac Cardiovasc Surg 2004;127:1188-92. [Crossref] [PubMed]
- Murthy NS, Maus TP, Behrns CL. Intraforaminal location of the great anterior radiculomedullary artery (artery of Adamkiewicz): a retrospective review. Pain Med 2010;11:1756-64. [Crossref] [PubMed]
- Kroszczynski AC, Kohan K, Kurowski M, et al. Intraforaminal location of thoracolumbar anterior medullary arteries. Pain Med 2013;14:808-12. [Crossref] [PubMed]
- Somayaji HS, Saifuddin A, Casey AT, et al. Spinal cord infarction following therapeutic computed tomography-guided left L2 nerve root injection. Spine (Phila Pa 1976) 2005;30:E106-8. [Crossref] [PubMed]
- Glaser SE, Falco F. Paraplegia following a thoracolumbar transforaminal epidural steroid injection. Pain Physician 2005;8:309-14. [PubMed]
- Quintero N, Laffont I, Bouhmidi L, et al. Transforaminal epidural steroid injection and paraplegia: case report and bibliographic review. Ann Readapt Med Phys 2006;49:242-7. [Crossref] [PubMed]
- Kennedy DJ, Dreyfuss P, Aprill CN, et al. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: two case reports. Pain Med 2009;10:1389-94. [Crossref] [PubMed]
- Wybier M, Gaudart S, Petrover D, et al. Paraplegia complicating selective steroid injections of the lumbar spine. Report of five cases and review of the literature. Eur Radiol 2010;20:181-9. [Crossref] [PubMed]
- Chang Chien GC, Candido KD, Knezevic NN. Digital subtraction angiography does not reliably prevent paraplegia associated with lumbar transforaminal epidural steroid injection. Pain Physician 2012;15:515-23. [PubMed]
- Tackla RD, Keller JT, Ernst RJ, et al. Conus medullaris syndrome after epidural steroid injection: Case report. Int J Spine Surg 2012;6:29-33. [Crossref] [PubMed]
- Raghavendra M, Patel V. Should we cease performing transforaminal injections? Reg Anesth Pain Med 2005;30:207-8; author reply 208-10. [Crossref] [PubMed]
- Bogduk N and International Spine Intervention Society. Standards Committee. Practice guidelines for spinal diagnostic and treatment procedures. 1st ed. San Francisco: International Spine Intervention Society 2004:347.
- Simon JI, McAuliffe M, Smoger D. Location of Radicular Spinal Arteries in the Lumbar Spine from Analysis of CT Angiograms of the Abdomen and Pelvis. Pain Med 2016;17:46-51. [PubMed]
- Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med 1991;58:159-64. [PubMed]
- Kambin P, Savitz MH. Arthroscopic microdiscectomy: an alternative to open disc surgery. Mt Sinai J Med 2000;67:283-7. [PubMed]
- Renfrew DL, Moore TE, Kathol MH, et al. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. AJNR Am J Neuroradiol 1991;12:1003-7. [PubMed]
- Furman MB, O'Brien EM, Zgleszewski TM. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine (Phila Pa 1976) 2000;25:2628-32. [Crossref] [PubMed]
- Denis I, Claveau G, Filiatrault M, et al. Randomized Double-Blind Controlled Trial Comparing the Effectiveness of Lumbar Transforaminal Epidural Injections of Particulate and Nonparticulate Corticosteroids for Lumbosacral Radicular Pain. Pain Med 2015;16:1697-708. [Crossref] [PubMed]
- Berthelot JM, Tortellier L, Guillot P, et al. Tachon's syndrome (suracute back and/or thoracic pain following local injections of corticosteroids). A report of 318 French cases. Joint Bone Spine 2005;72:66-8. [Crossref] [PubMed]
- Hajjioui A, Nys A, Poiraudeau S, et al. An unusual complication of intra-articular injections of corticosteroids: Tachon syndrome. Two case reports. Ann Readapt Med Phys 2007;50:721-3, 718-20.
- Berthelot JM, Le Goff B, Maugars Y. Side effects of corticosteroid injections: what's new? Joint Bone Spine 2013;80:363-7. [Crossref] [PubMed]
Contributions: (I) Conception and design: GM Lagemann; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: RK Yu, GM Lagemann, V Agarwal; (V) Data analysis and interpretation: A Ghodadra; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.


