Duchenne muscular dystrophy: the management of scoliosis
Introduction
Duchenne muscular dystrophy (DMD) is one of the most common muscular dystrophies affecting an estimated 1 in every 3,600–6,000 newborn males (1). In boys with DMD, progressive muscle weakness leads to loss of ambulation, scoliosis, respiratory deterioration and cardiac compromise. In this review we discuss the features and management of scoliosis in DMD.
Pathophysiology and pattern of presentation
DMD is an X-linked recessive disorder caused by a mutation in the gene encoding the protein dystrophin (2). These mutations lead to the loss of functional dystrophin. This loss of dystrophin affects the stability of the cell membrane in skeletal muscle cells and results in progressive muscle damage and dysfunction.
DMD has a spectrum of severity, with boys becoming symptomatic between 1 and 5 years of age. The diagnosis is most frequently made over the age of 4 years (3). Most commonly, boys present with a concern regarding motor function; other presentations include global developmental delay, failure to thrive, rhabdomyolysis following a general anaesthetic or with an incidental finding of raised creatine kinase or transaminase.
The classical presentation of DMD is observed with the child demonstrating a delay in achieving motor milestones [affected boys cannot run or jump and 50% start to walk after the age of 18 months (4)] and the classically described Gowers’ sign whereby patients need to support themselves with their hands on their thighs to help them rise from the floor (5).
The diagnosis is confirmed through genetic analysis of a blood sample for a mutation in the dystrophin gene. A muscle biopsy to confirm the absence of dystrophin may be used in addition where there is diagnostic uncertainty between Becker Muscular Dystrophy and DMD (for instance in children presenting at a young age with good motor skills), but genetic analysis enables appropriate counselling and the consideration of mutation-specific therapies (5).
As the child grows, their ability to walk deteriorates with stairs becoming more difficult. Toe walk with an increasingly lordotic gait is observed and eventually this will progress to complete loss of ambulation.
Ambulation is lost between the ages of 6 and 12 years with a median of 9.5 years (6). More recently this has been modified with the use of the steroid therapy, which slows progression and prolongs ambulation by 2 to 4 years in most boys. During the early stages of loss of ambulation, patients can maintain posture and self propel their wheelchair. However, as time progresses this ability is also lost.
The loss of ambulation heralds the start of the development of a scoliosis. As the scoliosis progresses it can cause a significant impact on the respiratory system. Lateral displacement and rotation of the vertebral bodies alters the mechanics and movement of the associated ribs during respiration. Furthermore, the abnormal shape of the thoracic cavity then puts the muscles of respiration at a disadvantage. Finally, the organs contained within the thoracic cavity are displaced and compressed. All of these combined factors mean that respiratory reserve is considerably reduced and worsens as the scoliosis progresses (7).
Separate to the changes in shape of the thoracic cavity, there is an effect on the heart as the loss of the dystrophin protein also affects cardiac muscle which leads to a dilated cardiomyopathy. Cardiac function in children and adolescents with DMD can be variable, but the natural history suggests that there is a drop in global cardiac function between 12–14 years of age (8,9). While respiratory failure remains the major cause of mortality, cardiac death does occur in approximately 15% of children (10).
As the child develops progressive scoliosis, they begin to demonstrate difficulty with positioning, and comfort in their wheelchairs due to the change in posture and development of pelvic obliquity. Ultimately adjustments to the chair, for the sake of comfort, cease to be enough to prevent breakdown the skin in the concavity of the scoliosis or on the buttocks from unequal weight transfer. In the past, this could lead to patients being entirely bed bound if a comfortable position within a chair could not be found (11).
Furthermore, scoliosis progression can cause costo-iliac impingement, a painful rubbing of the ribs against the iliac crest on the concavity of the curve (12). The development of this symptom, along with skin breakdown and general discomfort in a wheelchair all significantly impact on the child’s quality of life.
Scoliosis development in DMD
The reason for scoliosis development in patients with DMD is poorly understood.
The consensus appears to be that poor mobility and increasing muscle weakness leads to changes in trunk and eventually a progressive collapsing scoliosis (5,13).
In DMD patients the progression of scoliosis is rapid with an increase in angulation of between 16° and 24° per year, which often occurs fastest during the adolescent growth spurt (14).
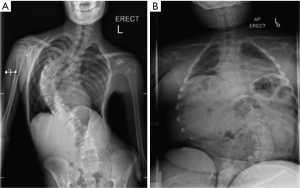
The shape of a scoliosis in DMD differs to that seen in adolescent idiopathic scoliosis as seen in Figure 1. The apex develops at the thoracolumbar junction of the spine and progression involves the whole thoracic and lumbar spine, leading to development of pelvic obliquity. Patients with DMD also appear to develop thoracolumbar kyphosis as opposed to the lordosis normally seen in idiopathic scoliosis (15).
Interventions before surgery
Attempts to prevent scoliosis are an important area of DMD management, however, once scoliosis has developed, surgical correction is the only solution. Historically attempts at bracing the spine to prevent progressive scoliosis have proven ineffective (14), though recent orthotic and wheelchair developments have improved DMD management. Unfortunately these postural alterations do not impact on the scoliosis once it has developed.
The importance of a multidisciplinary team approach has never been more important, with input from physiotherapists, rehabilitation specialists, orthopaedics, paediatrics, cardiology and respiratory medicine all playing a role in this patient group (16).
Improving patient comfort is important and includes postural adaptation, in particular inducing a lumbar lordosis by using a ‘wedge’ on a patient’s seat. This allows patients to tolerate a better lateral load and helps to prevent low back pain from a slumped position. Unfortunately this does not have any effect on the development of the scoliosis in the long term (17).
Maintaining ambulation for as long as possible can reduce the risk of the development of scoliosis and can decrease the severity of the scoliosis in the long term (18). Again, a multidisciplinary approach is essential involving physiotherapists, rehabilitation specialists and orthopaedic surgeons (19). Orthosis can be beneficial in helping to prevent the progression of lower limb contractures (20), but surgical management to release contractures may be necessary. There is no single procedure which all patients will require and therefore decisions over surgery must be on an individual patient basis.
Probably the most important development in overall DMD management has been the use of steroids. There is evidence that using steroids in patients with DMD can have numerous positive effects including prolonged independent ambulation, decreased progression of scoliosis, increased muscle strength, respiratory function, cardiac function and quality of life (18,21-26). The use of steroid therapy has been associated with a reduction in the incidence of scoliosis in DMD because prolonging ambulation means that some boys will undergo their pubertal growth spurt before loss of ambulation. There is also evidence that suggests patients treated with long-term steroids may avoid developing a scoliosis (24).
A number of studies have been performed comparing steroid administration to controls. One such study looked at a number of factors that might influence scoliosis development including age at onset, glucocorticoid treatment, age at loss of ambulation and forced vital capacity. In a cohort of 123 patients with DMD, the administration of prednisolone reduced the development of scoliosis, prolonging ambulation and a smaller scoliosis at 17 years of age (18).
The use of differing treatment regimens and medications exists within these studies (21,25,26); however, the overall results appear positive regardless of the regimen used. There is little consensus on the exact dose of steroids required. There is some guidance which can be of benefit in choosing an appropriate regimen (5) and, as suggested by the latest Cochrane review on DMD (27), there is an ongoing NIH-funded study comparing different steroid regimens which should provide a consensus on the best treatment (28).
While the use of steroids has been shown to have significant benefit in DMD patients, there are associated risks. All boys with DMD are osteoporotic, before initiation of steroid treatment. This is associated with a two-fold increased risk of long bone fractures compared to age matched controls (29). There is also a risk of vertebral compression fractures related to cumulative dose for those boys on steroid treatment (30). Other potential complications include the development of cataracts (24,26), the risk of spine and long bone fractures (22,25), and a decreased bone density (25). With regards to cataracts, both articles, while reporting differing incidence rates of cataracts, noted that those that required surgical correction for this was minimal (24,26).
The studies that reference low bone density and skeletal fractures have suggested regular dual energy x-ray absorptiometry (DEXA) scans for monitoring purposes. This, alongside calcium and vitamin D supplementation (22), as well as dietary advice and bisphosphonates (25) are adjuvant therapies which can help to reduce the risk of these complications.
Timing of surgery
Deciding when to intervene surgically for a scoliosis in DMD is challenging. At its core, surgery is performed to minimise and balance a scoliosis. This is to improve sitting position and comfort, reduce vertebral fractures and any associated pain and possibly to slow the rate of respiratory compromise (31).
However, the decision over when to operate is complex as not all patients develop scoliosis at the same time or have similar rate of progression. It has been suggested that although the majority of patients will require surgery, not all do (32). In these patients, use of a risk prediction system can help to decide which patients require early intervention and which patients intervention can be delayed (33).
Early surgery is essential in some patients who either have a rapid progression of their scoliosis, or whose respiratory and cardiac function are such that it can be anticipated that later surgery will not be viable. In those at high risk of a rapid deterioration, it is suggested that surgery should be performed once the curve progresses beyond 20° (34). In some patients, surgery may not be required if at skeletal maturity, the rate of scoliosis progression plateaus (24).
If a patient is deemed likely to require surgery, a forced vital capacity (FVC) less than 35% predicted predicts poor post-operative outcomes (35), due to a reduced reserve and an increased risk of respiratory compromise. This is not necessarily a contraindication to surgery but does suggest that complications are more likely and that additional ventilatory support may be required post-operatively (35). More recent research has suggested that if BIPAP is used post-operatively, even in those patients with a low FVC, there is a decrease in the time ventilatory support is required with no increase in the re-intubation rate (36). There is a role for pre-operative breathing exercises for six weeks before surgery (37). This increased patients’ FVC and even those with an FVC of less than 30% predicted at the time of surgery had no respiratory complications in the post-operative period in this series (37).
The assessment of any compromise in cardiac function is an important consideration before any surgical intervention can occur. Current guidelines on cardiac monitoring in DMD patients advocate specialist evaluation and input from the time of diagnosis (5,38). However, in a recent natural history study of DMD patients, cardiomyopathy was still under diagnosed and under-treated (8). A diagnosis of heart failure can be made in 38% of patients at 14 years of age, and 81% at 18 years of age [left ventricular (LV) ejection fraction <55% or shortening fraction <28%] (8). Current practice is to consider starting medication by 10 years of age, and that earlier initiation can prevent or delay LV dysfunction and reduce mortality (39,40). Angiotensin converting enzyme (ACE) inhibitors or Angiotensin II receptor blockers (ARBs) are equally effective in this setting (41). A combination of beta-blockers and ACE inhibitors/ARBs is used to maintain cardiac function; the use of beta blockers suggests a benefit in some retrospective (42,43) and prospective open, non-randomized (44,45) studies but not in others (46).
There is increasing debate about the extent and nature of cardiac imaging, including the use of cardiac MRI (8). In practice, however, the duration of the scan and the anaesthetic requirement preclude the routine widespread use in children and adolescents. The use of detailed echocardiogram can usually be of benefit in most children and adolescents. During the planning for scoliosis surgery, cardiac function should be taken into consideration. One of the authors (AC) uses as an ejection fraction of <40% as a cut off for the lower limit of surgery as it is felt that below this there is an unacceptable increase in mortality.
If the patient is on beta-blockers, and/or ACE inhibitors, these need to be taken into account pre-operatively due to blunting of sympathomimetic responses and hypotension. If beta blockade needs to be stopped pre-operatively this should be done in a phased manner over a few weeks, and then restarted in the post-operative period. The same should apply for ACE inhibitors, though they can be stopped a couple of days prior to the operation. Once the immediate high-risk post-operative period has passed, the beta-blockers and routine medication should be restarted.
Anaesthetic considerations
Children with DMD present a number of anaesthetic issues. These include poor respiratory reserve, cardiac dysfunction, the potential for major blood loss and anaesthetic drug issues.
Optimization of respiratory function before surgery is essential with the consideration of non-invasive ventilation and assisted cough techniques when appropriate (16,47).
The high incidence of cardiac dysfunction, both in terms of reduced contractility and arrhythmias, means that these patients may rapidly decompensate during induction of anaesthesia or with hypovolemia.
Due to the anaesthetic agent induced vasodilation, fluid shifts and blood loss, maintaining cardiac output can be challenging (38). Central venous and arterial blood pressure monitoring are essential to monitor haemodynamic status, and trans-oesophageal doppler or echocardiography may also be of use (48). Inotropes, such as dobutamine or dopamine, should be available.
Managing intravascular fluid volume is further complicated by the significant blood loss seen in DMD patients during scoliosis correction surgery, quoted as between 2.5–4.1 litres on average (14). This high level of blood loss is caused by poor contractility of dystrophic skeletal muscle and by and smooth muscle in the walls of the blood vessels within that muscle (14). There are a number of methods that can be used to try to reduce this blood loss including controlled hypotension, patient positioning, optimisation of coagulation and cell salvage.
Controlled hypotensive anaesthesia will reduce blood loss compared to normal anaesthesia techniques (49), but may not be tolerated in the presence of a cardiomyopathy. In addition, a low intra-operative mean arterial pressure (MAP) is associated with acute kidney injury, and can cause significant post-operative morbidity (50).
Correct positioning of the patient is essential as poor positioning, with abdominal compression, will exacerbate blood loss, thereby increasing the likelihood of hypovolemia and the development of a coagulopathy (51).
The use of tranexamic acid in DMD patients who are undergoing scoliosis surgery has been shown to reduce blood loss (51). In addition, prompt correction of a coagulopathy will reduce both intraoperative and postoperative bleeding.
Cell-saver technology is widely used in spinal surgery to collect blood from the operative field, and in some centres postoperatively, and then re-infuse back to the patient. This has been shown to reduce the number of blood transfusions required when compared to those who did not use cell-saver technology (52).
Despite the use of the above blood sparing techniques, blood transfusion can be required. In view of the reduced cardiac output, the risk of respiratory muscle fatigue and the likelihood of postoperative bleeding, one of the authors (AT) aims for a haemoglobin of greater than 100 g/L at the end of surgery.
DMD has an impact on the choice of anaesthetic agents, as some agents may destabilize the muscle membranes, particularly in young children where the rate of muscle regeneration is high. The depolarizing muscle relaxant suxamethonium is absolutely contraindicated, due its association with rhabdomyolysis and potentially fatal hyperkalaemia. Malignant hyperthermia does not occur in DMD but a similar syndrome of anaesthetic induced rhabdomyolysis (AIR) is well recognised and is associated, albeit rarely, with the use of volatile anaesthetic agents, such as isoflurane (53). However, this is less common in older children with DMD, as there is less muscle regeneration. Therefore although volatile anaesthetic agents are still used in these children, there is a move towards the use of total intravenous anaesthesia (TIVA), as there is significantly less risk with this method of anaesthesia (16,47,53).
Due to these considerations, pre-operative assessment of DMD patients must be robust. Upon completion of this assessment, discussion of the specific anaesthetic risks with the patient and the family prior to the procedure is important. These should include the risks of requiring prolonged postoperative ventilation, the need for a tracheostomy, cardiac arrest and death.
Surgical techniques
The technique used for scoliosis surgery in DMD has evolved over time as approaches and equipment have improved (15). There are a number of considerations regarding surgery including the approach, the type of metal to use, the limits of proximal and distal fusion, patterns of instrumentation and the type and source of bone graft.
An anterior approach to the spine is not advocated in DMD patients as it can require lung deflation leading to respiratory complications and a higher blood loss when compared to a posterior only approach (54).
Titanium is now the most commonly used metal for the manufacture of spinal implants used in this setting. The infection rate is reduced compared to steel implants, as the self-oxidising properties of titanium makes it relatively resistant to bacterial colonisation (55).
The level of proximal fusion is questioned across the literature. It is reported that the fusion needs to extend to T2 to prevent proximal junctional kyphosis developing (15). In our unit, however, the authors anecdotally support proximal fusion stopping at T6 in selected cases as this can maintain head control and allow continued self feeding, while also reducing theatre time, as it is a less extensive fusion (56).
The extent of distal fusion is also controversial. Our centre supports fusions to a distal level of L5 and this is supported by a study showing that in patients with minimal pelvic obliquity and scoliosis at the time of operation, fusions extending distally to L5, had a good outcome at 34 month follow up (57). It is reported, however, that patients who undergo fusion to L5 have increasing pelvic obliquity at 2 year follow up, but only 2 patients in this study required additional revision surgery for this (58). It has also been highlighted that better correction and maintenance of pelvic obliquity was not seen in those instrumented down to the sacrum compared with more proximal fusion to L5 (59). Differences within practice still exist and there is no clear evidence for which method gives the best long-term outcomes. However, the benefit of shorter proximal fusions, in terms of reduced theatre time while still providing good outcomes, would seem to point towards this as the appropriate method for correction.
Over the past 40 years different instrumentation techniques involving sublaminar wiring, hooks and pedicle screws have been used. During the early stages of pedicle screw use, there was evidence that their technical difficulty to insert, especially into osteopenic bone, led to higher rates of complications compared to a sublaminar alone system (60).
This led to the development of the hybrid method using both techniques which showed improved outcomes (60,61).
As time has progressed pedicle screws have become the widely accepted first choice. This is because their insertion does not require opening the spinal canal, which in itself provides a further operative risk to the patient. One study comparing three methods of instrumentation for the spine showed that the use of sublaminar wiring appears to increase theatre time and blood loss when compared to either pedicle screw only systems or a hybrid of both (61).
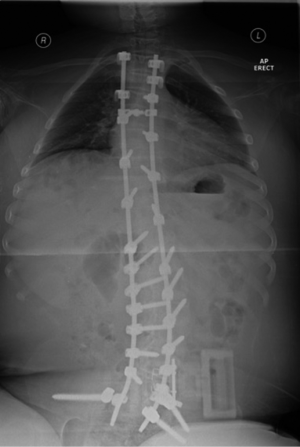
Pedicle screw only correction, as seen in Figure 2, has now been shown to be effective and it appears to provide good correction while also allowing early mobilisation after surgery reducing development of respiratory complications (62).
The use of autogenous iliac crest bone graft for fusion remains the gold standard for spinal fusion. Unfortunately, the collection of autogenous bone can cause significant complications, the most frequent being pain at the site of bone harvest. One article suggests the role of allograft bone, as this reduces the risks associated with autogenous bone collection but still provides the required osteoinductive activity (63). A more recent review article highlighted the move within spinal surgery to using demineralised bone matrix as this removes the donor site morbidity while still providing a satisfactory fusion (64).
Risks and complications
While surgery can provide benefits, there are also risks and complications. The cause of respiratory and cardiac risks have already been discussed but these risks include respiratory failure, aspiration, collapse of major lung segments, postoperative pneumonia, congestive heart failure, and cardiac arrhythmias. There are also reports of sudden cardiac arrest intra-operatively which are attributed to the underlying cardiomyopathy, the mechanism of which is still poorly understood (38,65).
There is a reported risk of wound infections (66,67). The recent use of local vancomycin powder in to the wound on top of the implant and bone graft appears to have a role in reducing the incidence of wound infections. A recent meta-analysis, confirms the effectiveness of local vancomycin powder application (68), and its safety in paediatric patients undergoing spinal fusion has been confirmed (69).
Pseudoarthrosis is a risk for any spinal fusion procedure and this can lead to rod breakage and subsequent deterioration of the scoliosis requiring revision surgery (70). The continuing development of new surgical techniques with three column rigid fixation using pedicle screws and the stimulation and development of a well formed fusion mass have reduced the rates of pseudoarthrosis significantly.
Loss of head control can be problematic, especially if during surgery the centre of mass of the head is moved backwards. The relative weakness in the neck flexors compared to the neck extensors leads to a loss of head control and a sagittal profile (71,72), requiring further orthotic or wheelchair support
There is also a risk of weight loss after surgery and this has been linked to a loss of ability to self feed secondary to the surgery (although this ability may be lost prior to surgery as part of disease progression). One study hypothesised that the loss of the ability to self feed was linked to the reduced range of neck movement and loss of head control (56). Another study found that the loss of ability to self feed was only present in those with significant scoliosis and difficulty sitting (73). Therefore, appropriate care during surgical correction of scoliosis to prevent loss of head control and supportive changes to the environment after surgery are thought to be the best way to prevent this complication.
Outcomes
Despite the risks associated with surgery, it delivers significant improvement in patients’ lives. Qualitative data studying the impact of surgical correction for patients and parents showed that surgery had improved function, sitting balance and quality of life (74).
The long-term effects of surgery on respiratory function are controversial as different researchers have found differing results. Several studies exist which show no improvement in respiratory function post-operatively and no change in respiratory deterioration (58,65,75,76). One long term study completed a 7-year follow up showing equal deterioration in respiratory function between controls and those who underwent surgery (77). There are studies which show an reduced rate of respiratory deterioration after spinal surgery (78,79), however, the overall consensus appears to be that surgery does not improve respiratory function or slow its deterioration. The use of steroids, improved respiratory care and non-invasive ventilation have all helped to maintain respiratory function long term (80). There is also evidence that life is prolonged in those who had both scoliosis surgery and used non-invasive ventilation (81).
Conclusions
DMD is a progressive condition which impacts on patients’ quality and quantity of life. This review has covered the current thoughts on the aetiology, presentation and management of scoliosis as it presents as part of DMD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bushby K. Duchenne muscular dystrophy factsheet.pdf. Muscular Dystrophy Campaign; 2012.
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919-28. [Crossref] [PubMed]
- van Ruiten HJ, Straub V, Bushby K, et al. Improving recognition of Duchenne muscular dystrophy: a retrospective case note review. Arch Dis Child 2014;99:1074-7. [Crossref] [PubMed]
- Bushby KM, Hill A, Steele JG. Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet 1999;353:557-8. [Crossref] [PubMed]
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77-93. [Crossref] [PubMed]
- Emery AE. The muscular dystrophies. Lancet 2002;359:687-95. [Crossref] [PubMed]
- Koumbourlis AC. Scoliosis and the respiratory system. Paediatr Respir Rev 2006;7:152-60. [Crossref] [PubMed]
- Spurney C, Shimizu R, Morgenroth LP, et al. Cooperative International Neuromuscular Research Group Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle Nerve 2014;50:250-6. [Crossref] [PubMed]
- Kirchmann C, Kececioglu D, Korinthenberg R, et al. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatr Cardiol 2005;26:66-72. [Crossref] [PubMed]
- Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th-9th June 2002, Naarden, the Netherlands. Neuromuscul Disord 2003;13:166-72. [Crossref] [PubMed]
- Smith AD, Koreska J, Moseley CF. Progression of scoliosis in Duchenne muscular dystrophy. J Bone Joint Surg Am 1989;71:1066-74. [PubMed]
- Wynne AT, Nelson MA, Nordin BE. Costo-iliac impingement syndrome. J Bone Joint Surg Br 1985;67:124-5. [PubMed]
- Hsu JD, Quinlivan R. Scoliosis in Duchenne muscular dystrophy (DMD). Neuromuscul Disord 2013;23:611-7. [Crossref] [PubMed]
- Moe JH. Moe's Textbook of Scoliosis and Other Spinal Deformities. 1995. Available online: https://books.google.co.uk/books?id=GoZsAAAAMAAJ
- Karol LA. Scoliosis in patients with Duchenne muscular dystrophy. J Bone Joint Surg Am 2007;89 Suppl 1:155-62. [Crossref] [PubMed]
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177-89. [Crossref] [PubMed]
- Kerr TP, Lin JP, Gresty MA, et al. Spinal stability is improved by inducing a lumbar lordosis in boys with Duchenne Muscular Dystrophy: a pilot study. Gait Posture 2008;28:108-12. [Crossref] [PubMed]
- Kinali M, Main M, Eliahoo J, et al. Predictive factors for the development of scoliosis in Duchenne muscular dystrophy. Eur J Paediatr Neurol 2007;11:160-6. [Crossref] [PubMed]
- Vignos PJ, Wagner MB, Karlinchak B, et al. Evaluation of a program for long-term treatment of Duchenne muscular dystrophy. Experience at the University Hospitals of Cleveland. J Bone Joint Surg Am 1996;78:1844-52. [PubMed]
- Hyde SA. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscul Disord 2000;10:257-63. [Crossref] [PubMed]
- Yilmaz O, Karaduman A, Topaloğlu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur J Neurol 2004;11:541-4. [Crossref] [PubMed]
- King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology 2007;68:1607-13. [Crossref] [PubMed]
- Harvey A, Baker L, Williams K. Non-surgical prevention and management of scoliosis for children with Duchenne muscular dystrophy: what is the evidence? J Paediatr Child Health 2014;50:E3-9. [Crossref] [PubMed]
- Lebel DE, Corston JA, McAdam LC, et al. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am 2013;95:1057-61. [Crossref] [PubMed]
- Houde S, Filiatrault M, Fournier A, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol 2008;38:200-6. [Crossref] [PubMed]
- Biggar WD, Politano L, Harris VA, et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscul Disord 2004;14:476-82. [Crossref] [PubMed]
- Cheuk DK, Wong V, Wraige E, et al. Surgery for scoliosis in Duchenne muscular dystrophy. Available online: http://doi.wiley.com/10.1002/14651858.CD005375.pub4
- Griggs R. Finding the Optimum Regimen for Duchenne Muscular Dystrophy (FOR-DMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01603407
- McDonald DG, Kinali M, Gallagher AC, et al. Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol 2002;44:695-8. [Crossref] [PubMed]
- Ricotti V, Ridout DA, Scott E, et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry 2013;84:698-705. [Crossref] [PubMed]
- Velasco MV, Colin AA, Zurakowski D, et al. Posterior spinal fusion for scoliosis in duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine (Phila Pa 1976) 2007;32:459-65. [Crossref] [PubMed]
- Kinali M, Messina S, Mercuri E, et al. Management of scoliosis in Duchenne muscular dystrophy: a large 10-year retrospective study. Dev Med Child Neurol 2006;48:513-8. [Crossref] [PubMed]
- Yamashita T, Kanaya K, Kawaguchi S, et al. Prediction of progression of spinal deformity in Duchenne muscular dystrophy: a preliminary report. Spine (Phila Pa 1976) 2001;26:E223-6. [Crossref] [PubMed]
- Shapiro F, Zurakowski D, Bui T, et al. Progression of spinal deformity in wheelchair-dependent patients with Duchenne muscular dystrophy who are not treated with steroids: coronal plane (scoliosis) and sagittal plane (kyphosis, lordosis) deformity. Bone Joint J 2014;96-B:100-5. [Crossref] [PubMed]
- Jenkins JG, Bohn D, Edmonds JF, et al. Evaluation of pulmonary function in muscular dystrophy patients requiring spinal surgery. Crit Care Med 1982;10:645-9. [Crossref] [PubMed]
- Harper CM, Ambler G, Edge G. The prognostic value of pre-operative predicted forced vital capacity in corrective spinal surgery for Duchenne's muscular dystrophy. Anaesthesia 2004;59:1160-2. [Crossref] [PubMed]
- Takaso M, Nakazawa T, Imura T, et al. Surgical management of severe scoliosis with high-risk pulmonary dysfunction in Duchenne muscular dystrophy. Int Orthop 2010;34:401-6. [Crossref] [PubMed]
- American Academy of Pediatrics Section on Cardiology and Cardiac Surgery. Cardiovascular health supervision for individuals affected by Duchenne or Becker muscular dystrophy. Pediatrics 2005;116:1569-73. [Crossref] [PubMed]
- Duboc D, Meune C, Lerebours G, et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 2005;45:855-7. [Crossref] [PubMed]
- Duboc D, Meune C, Pierre B, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years' follow-up. Am Heart J 2007;154:596-602. [Crossref] [PubMed]
- Allen HD. A randomized, double-blind trial of lisinopril and losartan for the treatment of cardiomyopathy in duchenne muscular dystrophy. PLoS Curr 2013.5. [PubMed]
- Jefferies JL, Eidem BW, Belmont JW, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation 2005;112:2799-804. [Crossref] [PubMed]
- Ogata H, Ishikawa Y, Ishikawa Y, et al. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol 2009;53:72-8. [Crossref] [PubMed]
- Matsumura T, Tamura T, Kuru S, et al. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Intern Med 2010;49:1357-63. [Crossref] [PubMed]
- Kajimoto H, Ishigaki K, Okumura K, et al. Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J 2006;70:991-4. [Crossref] [PubMed]
- Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 2007;298:1171-9. [Crossref] [PubMed]
- Birnkrant DJ, Panitch HB, Benditt JO, et al. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest 2007;132:1977-86. [Crossref] [PubMed]
- Cripe LH, Tobias JD. Cardiac considerations in the operative management of the patient with Duchenne or Becker muscular dystrophy. Paediatr Anaesth 2013;23:777-84. [Crossref] [PubMed]
- Fox HJ, Thomas CH, Thompson AG. Spinal instrumentation for Duchenne's muscular dystrophy: experience of hypotensive anaesthesia to minimise blood loss. J Pediatr Orthop 1997;17:750-3. [Crossref] [PubMed]
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013;119:507-15. [Crossref] [PubMed]
- Shapiro F, Zurakowski D, Sethna NF. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976) 2007;32:2278-83. [Crossref] [PubMed]
- Liang J, Shen J, Chua S, et al. Does intraoperative cell salvage system effectively decrease the need for allogeneic transfusions in scoliotic patients undergoing posterior spinal fusion? A prospective randomized study. Eur Spine J 2015;24:270-5. [Crossref] [PubMed]
- Muenster T, Mueller C, Forst J, et al. Anaesthetic management in patients with Duchenne muscular dystrophy undergoing orthopaedic surgery: a review of 232 cases. Eur J Anaesthesiol 2012;29:489-94. [Crossref] [PubMed]
- Suh SW, Modi HN, Yang J, et al. Posterior multilevel vertebral osteotomy for correction of severe and rigid neuromuscular scoliosis: a preliminary study. Spine (Phila Pa 1976) 2009;34:1315-20. [Crossref] [PubMed]
- Glotzbecker MP, Riedel MD, Vitale MG, et al. What's the evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop 2013;33:479-87. [Crossref] [PubMed]
- Iannaccone ST, Owens H, Scott J, et al. Postoperative malnutrition in Duchenne muscular dystrophy. J Child Neurol 2003;18:17-20. [Crossref] [PubMed]
- Mubarak SJ, Morin WD, Leach J. Spinal fusion in Duchenne muscular dystrophy--fixation and fusion to the sacropelvis? J Pediatr Orthop 1993;13:752-7. [Crossref] [PubMed]
- Alman BA, Kim HK. Pelvic obliquity after fusion of the spine in Duchenne muscular dystrophy. J Bone Joint Surg Br 1999;81:821-4. [Crossref] [PubMed]
- Gaine WJ, Lim J, Stephenson W, et al. Progression of scoliosis after spinal fusion in Duchenne's muscular dystrophy. J Bone Joint Surg Br 2004;86:550-5. [PubMed]
- Modi HN, Suh SW, Hong JY, et al. Treatment and complications in flaccid neuromuscular scoliosis (Duchenne muscular dystrophy and spinal muscular atrophy) with posterior-only pedicle screw instrumentation. Eur Spine J 2010;19:384-93. [Crossref] [PubMed]
- Arun R, Srinivas S, Mehdian SM. Scoliosis in Duchenne's muscular dystrophy: a changing trend in surgical management: a historical surgical outcome study comparing sublaminar, hybrid and pedicle screw instrumentation systems. Eur Spine J 2010;19:376-83. [Crossref] [PubMed]
- Hahn F, Hauser D, Espinosa N, et al. Scoliosis correction with pedicle screws in Duchenne muscular dystrophy. Eur Spine J 2008;17:255-61. [Crossref] [PubMed]
- Nakazawa T, Takaso M, Imura T, et al. Autogenous iliac crest bone graft versus banked allograft bone in scoliosis surgery in patients with Duchenne muscular dystrophy. Int Orthop 2010;34:855-61. [Crossref] [PubMed]
- Tilkeridis K. Use of demineralized bone matrix in spinal fusion. World J Orthop 2014;5:30-7. [Crossref] [PubMed]
- Shapiro F, Sethna N, Colan S, et al. Spinal fusion in Duchenne muscular dystrophy: a multidisciplinary approach. Muscle Nerve 1992;15:604-14. [Crossref] [PubMed]
- Heller KD, Wirtz DC, Siebert CH, et al. Spinal stabilization in Duchenne muscular dystrophy: principles of treatment and record of 31 operative treated cases. J Pediatr Orthop B 2001;10:18-24. [PubMed]
- Ramirez N, Richards BS, Warren PD, et al. Complications after posterior spinal fusion in Duchenne's muscular dystrophy. J Pediatr Orthop 1997;17:109-14. [Crossref] [PubMed]
- Chiang HY, Herwaldt LA, Blevins AE, et al. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J 2014;14:397-407. [Crossref] [PubMed]
- Armaghani SJ, Menge TJ, Lovejoy SA, et al. Safety of topical vancomycin for pediatric spinal deformity: nontoxic serum levels with supratherapeutic drain levels. Spine (Phila Pa 1976) 2014;39:1683-7. [Crossref] [PubMed]
- Thacker M, Hui JH, Wong HK, et al. Spinal fusion and instrumentation for paediatric neuromuscular scoliosis: retrospective review. J Orthop Surg (Hong Kong) 2002;10:144-51. [PubMed]
- Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg 2002;10:138-51. [Crossref] [PubMed]
- Granata C, Merlini L, Cervellati S, et al. Long-term results of spine surgery in Duchenne muscular dystrophy. Neuromuscul Disord 1996;6:61-8. [Crossref] [PubMed]
- Liu M, Mineo K, Hanayama K, et al. Practical problems and management of seating through the clinical stages of Duchenne's muscular dystrophy. Arch Phys Med Rehabil 2003;84:818-24. [Crossref] [PubMed]
- Takaso M, Nakazawa T, Imura T, et al. Surgical management of severe scoliosis with high risk pulmonary dysfunction in Duchenne muscular dystrophy: patient function, quality of life and satisfaction. Int Orthop 2010;34:695-702. [Crossref] [PubMed]
- Miller F, Moseley CF, Koreska J. Spinal fusion in Duchenne muscular dystrophy. Dev Med Child Neurol 1992;34:775-86. [Crossref] [PubMed]
- Miller F, Moseley CF, Koreska J, et al. Pulmonary function and scoliosis in Duchenne dystrophy. J Pediatr Orthop 1988;8:133-7. [Crossref] [PubMed]
- Kennedy JD, Staples AJ, Brook PD, et al. Effect of spinal surgery on lung function in Duchenne muscular dystrophy. Thorax 1995;50:1173-8. [Crossref] [PubMed]
- Galasko CS, Delaney C, Morris P. Spinal stabilisation in Duchenne muscular dystrophy. J Bone Joint Surg Br 1992;74:210-4. [PubMed]
- Suk KS, Lee BH, Lee HM, et al. Functional outcomes in Duchenne muscular dystrophy scoliosis: comparison of the differences between surgical and nonsurgical treatment. J Bone Joint Surg Am 2014;96:409-15. [Crossref] [PubMed]
- Finder JD, Birnkrant D, Carl J, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med 2004;170:456-65. [Crossref] [PubMed]
- Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy--the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord 2007;17:470-5. [Crossref] [PubMed]
Contributions: (I) Conception and design: JE Archer, AC Gardner; (II) Administrative support: None; (III) Provision of study materials or patients: JE Archer, AC Gardner; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.


