
Rural neurosurgical and spinal laboratory setup
Introduction
In recent years, there is growing interest and effort in increasing the role of simulation in surgical training in residency programs in the USA and other countries, and many neurosurgical and spinal surgical laboratories dedicated to conducting research and surgical simulation have been established. Such resources permit trainees to explore and understand surgical anatomy through cadaver dissection, and thus facilitating acquisition and development of fundamental surgical techniques in a safe and controlled environment.
The integration of microsurgical and endoscopic techniques in modern neurosurgery, as well as the introduction of new neuro-anatomical, neuropathological and technical concepts has highlighted the critical importance of acquiring advanced technical skills by residents.
Despite this ever-increasing level of knowledge and technical competence required of aspiring neurosurgical or spinal surgical trainees, the opportunities for hands-on cadaver training still remain limited in Australia. This has been attributed to the general abolition of dissection in the new medical curricula.
Further limits for the establishment of a conventional neurosurgical cadaver-dissection training facility comprise the considerable physical resources required for the maintenance of a functional neurosurgical dissection training laboratory, and particularly in Australia, the wide geographical dispersal of aspiring and in-training neurosurgical trainees, who would benefit from such a facility.
To this end, we report our experience in setting up an Australian cadaver-based neurosurgical research laboratory in Australian rural areas, demonstrating the feasibility of establishing a cost-effective anatomical research facility in the Australian setting.
As part of the surgical dissection course for junior surgical trainees offered by the University of New England (UNE), a neurosurgical laboratory was established within the University’s School of Rural Medicine’s anatomy facility. Resources required for a functioning elementary neurosurgical laboratory had an affordable cost and allowed for conducting several research projects simultaneously.
Materials and methods
Anatomy laboratory at the University of New England, Armidale
UNE Medical School is a newly founded rural medical school in Armidale, New South Wales (NSW), Australia. It conducts a rurally-delivered five-year undergraduate medical course in conjunction with the more established University of Newcastle Medical School (Joint Medical Program). The two metropolitan areas in proximity to UNE by car are Newcastle (5 hours) and Sydney (7 hours).
As part of the certified, and ethically approved, body donor program, the UNE anatomy laboratory receives regional rural donor cadavers. Specimens are delivered to the laboratory on the day of, or within a maximum of 5 days of death. They are then embalmed, using GenelynTM embalming fluid (10% formaldehyde) via a trans-femoral injection. The donated cadavers are used in an elective anatomy dissection course taken by UNE’s preclinical medical students, and an intensive 16-day postgraduate diploma for surgical trainees. The Master of Applied Anatomy by Dissection cadaver research is a research-focused extension of the diploma course.
Results
Acquisition of surgical equipment
Surgical and microsurgical instruments and a Zeiss OPMI Pico operating microscope were available through the senior author’s personal equipment (C.G.). The senior author (C.G.) also provided personal photographic tools including macro lens and ring flash, 3D video camera and recording equipment to digitalize microscope output.
Two high-speed drills, with a variety of drill bits were loaned by Stryker (Kalamazoo, MI). Operating endoscopes were instead loaned by Storz (Tubingen, Germany). Cautery was not required in this cadaveric setting, while neuronavigation could not be affordably attained.
Cadaver preparation
A number of setups were trialled before a satisfactory one was selected. Initially, out of a desire to maximize the experience for more students to benefit from the whole-body specimens, cadaver heads were not decapitated for surgery. Bodies were taped to the dissecting table and maneuvered for each operation.
We found this setup cumbersome and untenable. The GenelynTM-fixed cadaver permitted very limited flexion, extension or rotation of the head as required for adequate surgical positioning and exposure. Additionally, one of our projects entailed evaluating a surgical approach that required a semi-sitting position such as the cerebellum, which was not achievable with the rigid body.
Furthermore, bodies embalmed via the femoral vein always resulted in poorly preserved brains after surgical exposure. Very limited formaldehyde evidently perfused through to the cerebral tissue, and basic neurosurgical maneuvers such as soft retraction would often destroy the anatomy. Hence, we concluded that only decapitated heads could be appropriate for our purposes.
These heads were prepared within 24 hours upon arrival of the cadavers to the laboratory. Decapitation at the low-cervical level was performed. The common carotids were accessed, washed out, and then infused with formaldehyde. The specimens were left for several hours in a container of formaldehyde to consolidate.
Cerebrovascular colored injection
After attempts on two cadaver heads, we found the anatomical research and educational value of specimens to be in need of intracranial colored injection of blood vessels, as tiny veins could not be readily differentiated from arteries, nor blood vessels differentiated from nerves. We subsequently obtained colored silicon from a commercial arts and crafts store.
The six major vessels—the common carotid arteries, vertebral arteries, and internal jugular veins—were catheterized with indwelling catheters, and secured with sutures, as described by Alvernia et al. (Figure 1) (1). The major vessels were flushed continuously by hand with warm water until clear fluid returned. This typically took at least 1 to 2 hours.
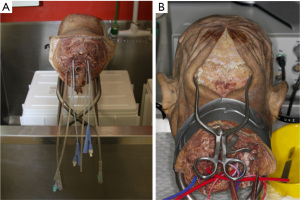
Leaks from the system were identified, and were managed with either ligation or glue. After flushing, the heads were left in formaldehyde overnight to allow for reduction of the swelling of soft tissue caused by continuous irrigation. Silicon injection under handheld syringe pressure with red dye for the arterial system and blue dye for the venous system then followed. We looked for passage of dye through the corresponding vessel to confirm correct passage; for example, red dye injected into the left common carotid extravasating out of the right common carotid and/or left vertebral artery. The catheters were then ligated, and the specimen allowed to set for 48 hours.
Surgical setup
An improvised Mayfield clamp was created using duct tape and string to immobilize the head onto crossbars which were then tied to the dissecting table. The crossbar configuration allowed for adjustable prone, supine, and semi-sitting positions, as well as rotation of the head.
Microdissection and retraction equipment was laid out. The operating microscope was secured to the table (Figure 2). The endoscope, monitor and high-speed drill were laid out to each side of the dissecting table. Suction apparatus was made available.

A note on improvisation—‘the Aldiscope’
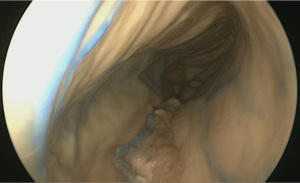
Drawing inspiration from surgical simulation laboratories, which have emulated laparoscopic surgery using economical digital cameras and a display monitor, we tested the feasibility of using a cheaply purchased borescope. This was an optical device consisting of a flexible tube with an eye piece at one end and a display monitor at the other. Typically, borescopes are used by plumbers, carpenters or mechanics to inspect regions, which are otherwise inaccessible. We wrapped the borescope with a fine bore catheter, which allowed us to flush and clean the camera lens intraoperatively (Figure 3). Having sourced this from a local ALDI shop, we dubbed the device ‘the Aldiscope’.

Although unsurprisingly, the Aldiscope could not provide anywhere near the same image resolution as the Storz endoscope, it was able to provide crude images of the interior of the cranium (Figure 4). Other drawbacks included its large diameter (10 mm). Its major advance, however, particularly for enthusiastic medical students, was its reasonable simulation of the neuro-endoscopic experience, rendering it a good training tool to practice endoscopic technical skills.
Quality of dissection
Prior to commencing the operation, a small cut over the superficial temporal artery was made to assess the quality of the silicon infiltration into the arterial tree. Usually the venous system could be assessed in a similar manner. The brains from specifically-prepared heads in the latter stages of our dissection were well preserved with good volume and consistency. We found the arterial system easier to fill with colored silicon than the venous; deep veins were often not reached. This was probably due to the high capacitance of the dural venous sinuses coupled with the rapid hardening times of the mixture. Later experience with latex injection system (purchased from Wards Natural Science) achieved superior results in the venous compartment. In some cases, extravasation of arterial silicon into the subarachnoid space was observed, probably due to the high pressures required for injection. In these cases, the research value of the specimens was greatly reduced.
Examples of surgical operations that were performed included placement of frontal and occipital ventriculostomy (under endoscopic vision) (Figure 4), bifrontal craniectomy, pterional craniotomy, mastoidectomy, endoscopic endonasal exposure, and the supracerebellar approach to the posterior cranial fossa.
Spine surgery equipment
Simulation of spine surgery was accomplished by the use of fresh sheep cadavers, which closely resemble human spine anatomy, as already validated training models. Animal cadavers were courtesy provided by the Large Animal Research and Imaging facilities at the Veterinary Services Field Station (Blacks Road, Gilles Plains, Adelaide, Australia) in collaboration with Department of ENT surgery of the Royal Adelaide Hospital, Adelaide, Australia (Figure 5).
Surgical equipment, as well as spine instrumentation, was loaned by Stryker (Kalamazoo, MI, USA) as stock material for teaching purposes.
A mobile X-ray imaging system (C-arm), loaned by a veterinary clinical practice, was used to acquire preoperative images. Imaging was reconstructed in the three dimensions for surgical planning and for preoperative anthropometric analysis, using Osirix DICOM viewer (Pixmeo SARL).
Animals were put in surgical position and dissected according to the selected surgical procedure. Skin incision was centered onto the target area under fluoroscopic vision; C-arm machine was intraoperatively further used for screw and cage placements, as in the real surgical practice.
For anterior approaches, due to anatomical reasons, sheep are preferred for trans-thoracic routes, while pigs are suggested for anterior trans-abdominal and lumbar lateral approaches. The main rationale to use pigs for trans-abdominal approaches is the anatomical conformation of abdominal organs, which more strictly resemble human anatomy, not comprising the rumen.
Endoscopic procedures were carried out using the same rigid endoscopes applied for skull base surgery training (Storz; Tubingen Germany). Skull base high-speed drill bits (Stryker; Kalamazoo, MI, USA) were assembled on long straight handpieces and used during endoscopic procedures.
Spine models of live surgery
A further advantage of the use of fresh cadavers is the possibility to test the functional integrity of white matter fiber tracts, after performing a median myelotomy or the dissection of a spinal cord intra-axial lesion. The motor response after surgery can be tested by electric stimulation, using the common electric stimulation lead of an electrician.
To simulate live surgery on cadaveric thoracic-lumbar spine model, fresh animal cadavers were used. Aorta was ligated cranially at the descending segment and caudally at the iliac bifurcation and catheterized with indwelling catheters, secured with sutures. Catheters were connected to a pump, commercially produced for plumbers, that injected red-colored saline water through the aorta into intercostal arteries and spinal branches. This setting, together with the use of fresh tissues, simulated live surgery in terms of bleeding, pulsation, and softness of tissue.
Ultimately, to train younger residents with watertight dural suture and leak repair, the tip of an irrigation pipe was placed subdurally, three levels cranially to a selected target area; subdural space was filled with plain SALF water, mimicking spinal fluid. A cut made at the selected target area simulated the dural tear, which was further repaired as training procedure. The same model was used by residents to practice performing lumbar puncture.
As already described for skull base surgery training, a further advancement of the tumor model was applied in spinal surgical training (2).
Spine model for anatomical research and development of innovative surgical tools
Spine cadaver models were used to develop innovative spine instrumentation. Fresh cadaver tissues, closely simulating real surgical anatomy, served as models to test safety as well as efficacy of this new prototype of surgical tools. New dissectors to perform lumbar flavectomy were developed. Life-surgery spinal fluid model was used to test instrument safety in preserving the underlying dural lining.
Also human cadaver trunks were utilized prior to anatomical dissections for the anatomy curriculum of the medical school to study the anatomy of the lumbar sympathetic trunk as it pertains to lateral access surgery for lumbar fusion.
Morphological studies were performed both on animal and human cadavers, describing surgical anatomy of the ligamentum flavum, considered as a critical structure in posterior approaches to spinal canal (3,4).
Discussion
In this paper, we described our experience in establishing an elementary but valuable neurosurgical laboratory from a standard anatomical facility at a medical school in rural Australia. This laboratory was able to provide trainees with hands-on practice and familiarity with highly specialized neurosurgical equipment, surgical skills, theater setup, common neurosurgical approaches, as well as an opportunity to explore intricate neuroanatomy.
We have acknowledged the generosity and cooperativeness of industry in Australia in supporting academic neurosurgical work and training. In addition, we have alluded to a handful of improvised and cost-effective solutions, where instrumentation may be lacking. Probably the most expensive conclusion we have reached, however, is the need for heads to be decapitated for the purposes of neurosurgical simulation. This is an unfortunate necessity, which we acknowledge may compromise the value of the remaining cadaver for other purposes and may hence be a prohibitive factor for those running general anatomical facilities.
In one of his articles, Rhoton has previously described the quintessential operative techniques and features of a state-of-the-art neurosurgical theatre (5). The paper provides an excellent review of fundamentals for the practicing neurosurgeon, but it may be challenging reading for the junior trainee or medical student unfamiliar with the tools and practicalities of this highly specialized field. Limited time available for the trainees at all levels and the complexity of the three-dimensional anatomy of the brain, as well as the peculiar characteristics of this through different surgical approaches, make it necessary for everyone involved in neurosurgical care to be familiar with the anatomy of the brain and skull.
Practical guidelines for the establishment of a world-class neurosurgical laboratory have previously been published. Salma et al. published a list of the basic requirements such a lab should have access to, including embalmed specimens, surgical instruments and equipment (6). Protocols were also provided detailing the proper methods of preparing, embalming, storing and dissecting specimens. Tschabitscher and Di Ieva expanded on this, by detailing the different practical areas in which a surgeon could be educated in an anatomical lab, including surgical simulation, anatomical orientation and a comparison of endoscopic and microsurgical techniques (7).
Unfortunately, establishing surgical simulators and laboratories may not be feasible due to its prohibitive cost for many institutions worldwide (6,7). In this context it is appropriate to use borrowed equipment or improvised measures to reduce costs. Pokorny and McLaren created laparoscopic trainer boxes from simple plastic tubs, saving 90% of the cost of a professionally manufactured trainer (8). In a similar fashion, we have suggested the use of the borescope, although improvements in irrigation within the brain are needed.
Berg et al. espoused surgical training using inexpensive models and expired material, and demonstrated that adopting these means resulted in a progressive decrease in the cost of teaching per surgical resident (9).
An interesting suggestion has been made by Martins and Montero, who recommended establishing a microsurgical lab adjacent to an animal care facility (10). They emphasized the importance of practicing on animal specimens before moving onto human subjects. Neurosurgical simulation using cow heads has been previously described (11). In our experience using fresh sheep cadavers after they had been used for other in-vivo teaching activities, enabled us to reduce the cost. Sheep are an already validated ex-vivo model in spine surgery training (12).
Another cheap alternative is to obtain animals (unused heads of cattle) from slaughters. Fresh tissues in spine surgery are mandatory, because they closely mimic the actual consistency of soft tissues while performing dissections. Animal cadavers are easily available in rural areas and provide a useful and cheap educational tool.
Cooperation with neighboring veterinary or agricultural facilities, which are widely available in rural areas, is a key point in setting up animal cadaver dissection projects. In our experience, intra-operative scopic vision was provided by a mobile C-arm device, loaned by a veterinary clinical practice.
Availability of pre-operative imaging enables trainees to discuss surgical cases before surgeries, tailoring the surgical approach according to local anatomy. Moreover, anthropometric measurements of pedicles on pre-operative X-ray imaging, allow surgeons to select preoperatively the most appropriate instrumentation.
Live surgery models in spinal surgery training with fresh cadavers are essential in acquiring technical skills for trainees, because they realistically represent intraoperative conditions in terms of bleeding, pulsation and spinal fluid circulation. Animal live spine surgery models, as described before, represent a user-friendly and cheap alternative to already-reported live surgery models applied to cranial neurosurgery (13). They enable trainees to deal with intraoperative bleeding from spinal vasculature as well as dural repair and lumbar tap techniques.
Finally from our experience, many medical students who became involved in the process of setting up and utilizing our laboratory later expressed their interest in exploring a neurosurgical career. Indeed, early exposure to neurosurgery in medical school promotes the attraction of the best medical students to the specialty and has been shown to increase subsequent resident applications (14,15). Whilst exposure to the specialty may include lectures, forums, special interest groups and early ‘shadowing’ of neurosurgeons (16), we propose that there is no better way than the hands-on laboratory-controlled approach to interest a wide array of pre-clinical students to the appeal of neurosurgery. This exposure will benefit many young clinicians especially those from rural medical schools, many of whom would have graduated without having rotated through a term in neurosurgery.
Resourcefully setting up an affordable neuroanatomical laboratory, as described in our study, allows neurosurgical simulation to be more widely available to aspiring trainees, providing them with the opportunity to develop their technical skills as well as fundamental knowledge.
Conclusions
The presence of a neurosurgical laboratory provides trainees with the opportunity to explore their craft and extend their knowledge. It also exposes many pre-clinical students to the awe of neurosurgery in a non-intimidating and supervised environment. A considerable financial hurdle and learning curve can be associated with the establishment of such a laboratory; these are some of the likely reasons hindering its widespread adoption in Australia.
However, here we have demonstrated the feasibility and value of pursuing this goal, and contributed some lessons learnt from our experiences. With the establishment of further laboratories such as our own, more aspiring neurosurgeons may be trained to the benefit of their future patients.
Acknowledgements
We would like to thank Stryker, Medtronic, Storz, and Zeiss for their generosity in facilitating our access to the neurosurgical equipment detailed in this article, without which our research would not have been possible.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alvernia JE, Pradilla G, Mertens P, et al. Latex injection of cadaver heads: technical note. Neurosurgery 2010;67:362-7. [PubMed]
- Gragnaniello C, Gagliardi F, Chau AM, et al. Intracranial injectable tumor model: technical advancements. J Neurol Surg B Skull Base 2014;75:301-8. [PubMed]
- Aboud E, Al-Mefty O, Yaşargil MG. New laboratory model for neurosurgical training that simulates live surgery. J Neurosurg 2002;97:1367-72. [PubMed]
- Chau AM, Seex K, Gragnaniello C. Reply to commentary on "lateral extent and ventral laminar attachments of the lumbar ligamentum flavum: cadaveric study". Spine J 2015;15:797. [PubMed]
- Rhoton AL Jr. Operative techniques and instrumentation for neurosurgery. Neurosurgery 2003;53:907-34; discussion 934. [PubMed]
- Salma A, Chow A, Ammirati M. Setting up a microneurosurgical skull base lab: technical and operational considerations. Neurosurg Rev 2011;34:317-26; discussion 326. [PubMed]
- Tschabitscher M, Di Ieva A. Practical guidelines for setting up an endoscopic/skull base cadaver laboratory. World Neurosurg 2013;79:S16.e1-7.
- Pokorny MR, McLaren SL. Inexpensive home-made laparoscopic trainer and camera. ANZ J Surg 2004;74:691-3. [PubMed]
- Berg DA, Milner RE, Fisher CA, et al. A cost-effective approach to establishing a surgical skills laboratory. Surgery 2007;142:712-21. [PubMed]
- Martins PN, Montero EF. Organization of a microsurgery laboratory. Acta Cir Bras 2006;21:187-9. [PubMed]
- Hicdonmez T, Hamamcioglu MK, Parsak T, et al. A laboratory training model for interhemispheric-transcallosal approach to the lateral ventricle. Neurosurg Rev 2006;29:159-62. [PubMed]
- Suslu HT, Tatarli N, Karaaslan A, et al. A practical laboratory study simulating the lumbar microdiscectomy: training model in fresh cadaveric sheep spine. J Neurol Surg A Cent Eur Neurosurg 2014;75:167-9. [PubMed]
- Chau AM, Pelze NR, Hampton J, et al. Basic Science Lateral extent and ventral laminar attachments of the lumbar ligamentum flavum: cadaveric study. Spine J 2014;14:2467-71. [PubMed]
- Brem H, Amundson E. Preparing Hopkins medical students for a career in academic neurosurgery. Surgery 2003;134:414-5. [PubMed]
- Hill CS, Dias L, Kitchen N. Perceptions of neurosurgery: a survey of medical students and foundation doctors. Br J Neurosurg 2011;25:261-7. [PubMed]
- Saleh M. Attracting the top medical students to a career in neurosurgery. Br J Neurosurg 2013;27:405. [PubMed]


