
Granular cell tumors of the spinal canal: intramedullary case report and a review of the literature
Introduction
Granular cell tumors (GCTs) are rare tumors that most commonly occur in the tongue, other areas of the head and neck, and the skin of the upper limbs and trunk. GCTs of the nervous system arise from peripheral nerves and the central nervous system (CNS), however only 11 cases have been reported in the spine (1-10). We here present the case of a 13-year-old girl with a GCT of the right C5 nerve root within the C4/5 foramen and review the published cases of spinal GCT.
Case presentation
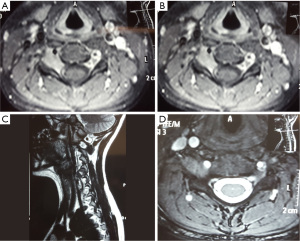
A 13-year-old girl presented to the neurosurgical outpatient department with a one-year history of neck and right shoulder pain associated with paresthesia. She reported the pain was exacerbated by injury one month prior to review and described limited abduction of her right shoulder associated with temporary sensory disturbance in the C5 dermatome which resolved spontaneously within a week. There was no family history of neurofibromatosis and the patient was previously well with no regular medications. Examination revealed reduced power (4+/5) of right shoulder abduction and reduced sensation to touch and pinprick over the right epaulette region. The rest of the neurological examination was normal. Magnetic resonance imaging (MRI) (Figure 1) demonstrated a mildly enhancing mass in the right C4/5 foramen extending up to the central canal. Expansion of the neural foramen suggested a chronic process and the lesion was thought to represent a sporadic benign peripheral nerve sheath tumor. As the patient’s symptoms failed to respond to conservative management surgical decompression of the nerve root was planned. A right C4/5 foraminotomy and core biopsy of the tumor was carried out and the tumor appeared to arise from the C5 nerve root. There were no postoperative complications and the patient reported improved right shoulder pain, however right shoulder abduction remained weaker. She was discharged on day 5 after surgery.
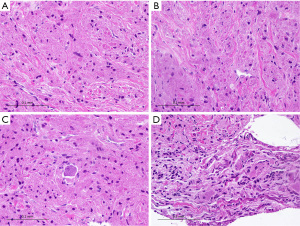
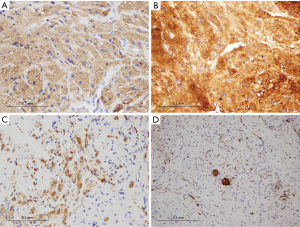
On histopathology (Figure 2), the biopsy specimen showed large, round to oval cells arranged in vague nests, showing abundant eosinophilic granular cytoplasm with intensely stained phagolysosomal globules frequently seen. The tumor cells were positive for S100 (diffuse), neuron-specific enolase (diffuse) and CD68 (patchy) (Figure 3). The findings were consistent with a GCT and the presence of nerve twigs and ganglion cells suggested a neural/ganglion origin. A decision was made against resection of the tumor given concerns about postoperative neurological deficit and a plan was made for conservative management with regular clinical review.
Discussion
GCTs are rare tumors that have a broad anatomical distribution, most commonly occurring in the head and neck (approximately 50% of all GCTs), particularly in the tongue (approximately 25%) (11). GCTs of the nervous system are rare, and may arise from peripheral nerves or the central nervous system (CNS). In the CNS, lesions have been reported in the cerebral hemispheres, cranial nerves and spinal cord, but most often occur in the neurohypophysis or infundibulum (12,13). GCTs arising in the spine are particularly rare and only 12 cases have been reported, including the patient presented above (1-10). Within this small subset of GCTs, there is variation in location of lesions with respect to the spinal cord, nerve roots and meninges. Nine cases (75%) involved the spinal nerve root and one case involved a dumbbell tumor of the C5/6 foramen. In one case partial nerve resection of small motor rootlets was required (8), while at least three cases required complete sacrifice of one or more nerves (5,6,9). Seven tumors were intradural-extramedullary with minimal or no involvement of the spinal cord itself, including the case reported above (1,6-10). In contrast, five tumors, appeared to originate from the nerve root or spinal cord itself (2-5). Some authors have suggested that an intramedullary component may represent extension of Schwannoma cell cytoplasm into the cord at the dorsal root zone (3), while others described an exophytic intramedullary tumor (4). The distinction between extramedullary tumors and those with intramedullary extension (or origin) may be clinically important and could help to define tumors that are amenable to resection without risk of neurological deficit.
In light of the limited literature to guide the management of this rare presentation, the optimal management of this case was not clear. However, due to concerns about postoperative neurological deficit, particularly as the lesion appeared to arise from within the C5 spinal nerve root, the plan was for conservative management with regular clinical review. Nerve root sacrifice has been studied in the context of spinal schwannoma surgery with variable rates of neurological morbidity, and these reports are limited to small case series (14-16). While it may be appropriate to manage extramedullary GCTs in a similar way, we would be reluctant to apply these findings to this case with a different tumor biology.
Debate regarding the cellular origin of GCTs continues. Despite being originally thought to be of muscular origin, GCTs are now considered a benign nerve sheath tumor on the basis of more recent immunohistochemical studies (17-19). Although this evidence suggests a Schwann cell origin, some authors propose that GCTs arising in the neurohypophyseal region of the CNS may be of pituicyte origin (8,12,13,20). In contrast, cerebral hemisphere lesions tend to be positive for glial fibrillary acidic protein (GFAP) which, in addition to ultrastructural features, is consistent with astrocytic origins (11,21-23). Granular cell change has been reported in various benign and malignant tumors of the CNS, such as granular cell astrocytomas, and should be distinguished from the ‘pure’ GCT due to different tumor biology and therefore clinical implications (12). GCT cells, typically polygonal or rounded, are arranged in nests or sheets, characteristically stain positive for S-100 protein, neuron-specific enolase and laminin, and the typical eosinophilic phagolysosomal granules express CD68 (24). The vast majority are benign neoplasms, with less than 2% found to be malignant, which have some characteristic morphological differences (17).
GCTs of the spine are intradural tumors that tend to involve one or more nerve roots. Lesions may be extramedullary and completely confined to the leptomeninges, while some have an intramedullary component and may arise from within the spinal cord or nerve. The latter may present a management challenge due to concerns about postoperative neurological deficit. All cases of GCT in this rare location should be contributed to the literature to help develop further understanding of this rare tumor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Markesbery WR, Duffy PE, Cowen D. Granular cell tumors of the central nervous system. J Neuropathol Exp Neurol 1973;32:92-109. [PubMed]
- Strömblad LG, Brun A, Cameron R, et al. Spinal granular cell tumor with subarachnoid hemorrhage: case report. Neurosurgery 1987;21:230-3. [PubMed]
- Burton BJ, Kumar VG, Bradford R. Granular cell tumour of the spinal cord in a patient with Rubenstein-Taybi syndrome. Br J Neurosurg 1997;11:257-9. [PubMed]
- Critchley GR, Wallis NT, Cowie RA. Granular cell tumour of the spinal cord: case report. Br J Neurosurg 1997;11:452-4. [PubMed]
- Haku T, Hosoya T, Hayashi M, et al. Granular cell tumor of the spinal nerve root: MR findings. Radiat Med 2002;20:137-40. [PubMed]
- Takayama Y, Hasuo K, Takahashi N, et al. Granular cell tumor presenting as an intradural extramedullary tumor. Clin Imaging 2004;28:271-3. [PubMed]
- Qu J, Ma J, Luo L, et al. Subdural granular cell tumor in thoracic vertebral canal. Neurol India 2009;57:679-81. [PubMed]
- Weinstein BJ, Arora T, Thompson LD. Intradural, extramedullary spinal cord granular cell tumor: a case report and clinicopathologic review of the literature. Neuropathology 2010;30:621-6. [PubMed]
- Lee CH, Hyun SJ, Lee JW, et al. Granular cell tumor of the intradural extramedullary spinal cord: report of two cases with respect to radiological differential diagnosis. J Korean Neurosurg Soc 2013;53:121-4. [PubMed]
- Vaghasiya VL, Nasit JG, Parikh PA, et al. Intradural spinal granular cell tumor. Asian J Neurosurg 2014;9:96-8. [PubMed]
- Ordóñez NG, Mackay B. Granular cell tumor: a review of the pathology and histogenesis. Ultrastruct Pathol 1999;23:207-22. [PubMed]
- Trimaldi J, Riddle ND, Bowers JW, et al. Granular tumors of the central nervous system: A case series. International Journal of Case Reports and Images 2013;4:1-6.
- Rickert CH, Kuchelmeister K, Gullotta F. Morphological and immunohistochemical characterization of granular cells in non-hypophyseal tumours of the central nervous system. Histopathology 1997;30:464-71. [PubMed]
- Kim P, Ebersold MJ, Onofrio BM, et al. Surgery of spinal nerve schwannoma. Risk of neurological deficit after resection of involved root. J Neurosurg 1989;71:810-4. [PubMed]
- Satoh N, Ueda Y, Koizumi M, et al. Assessment of pure single nerve root resection in the treatment of spinal schwannoma: focus on solitary spinal schwannomas located below the thoracolumbar junction. J Orthop Sci 2011;16:148-55. [PubMed]
- Celli P. Treatment of relevant nerve roots involved in nerve sheath tumors: removal or preservation? Neurosurgery 2002;51:684-92; discussion 692. [PubMed]
- Fanburg-Smith JC, Meis-Kindblom JM, Fante R, et al. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779-94. [PubMed]
- Mukai M. Immunohistochemical localization of S-100 protein and peripheral nerve myelin proteins (P2 protein, P0 protein) in granular cell tumors. Am J Pathol 1983;112:139-46. [PubMed]
- Smolle J, Konrad K, Kerl H. Granular cell tumors contain myelin-associated glycoprotein. An immunohistochemical study using Leu 7 monoclonal antibody. Virchows Arch A Pathol Anat Histopathol 1985;406:1-5. [PubMed]
- Schaller B, Kirsch E, Tolnay M, et al. Symptomatic granular cell tumor of the pituitary gland: case report and review of the literature. Neurosurgery 1998;42:166-70; discussion 170-1. [PubMed]
- Lee D, Suh YL. Nam do H. Cerebral granular cell tumor. Neuropathology 2008;28:417-21. [PubMed]
- Dickson DW, Suzuki KI, Kanner R, et al. Cerebral granular cell tumor: immunohistochemical and electron microscopic study. J Neuropathol Exp Neurol 1986;45:304-14. [PubMed]
- Nishioka H, Ii K, Llena JF, et al. Immunohistochemical study of granular cell tumors of the neurohypophysis. Virchows Arch B Cell Pathol Incl Mol Pathol 1991;60:413-7. [PubMed]
- Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss's Soft Tissue Tumors. 6th ed. Benign Tumours of Peripheral Nerves; Philadelphia: Elsevier; 2014:784-854. Available online: http://www.us.elsevierhealth.com/pathology/enzinger-and-weiss-soft-tissue-tumors-expert-consult/9780323088343/


